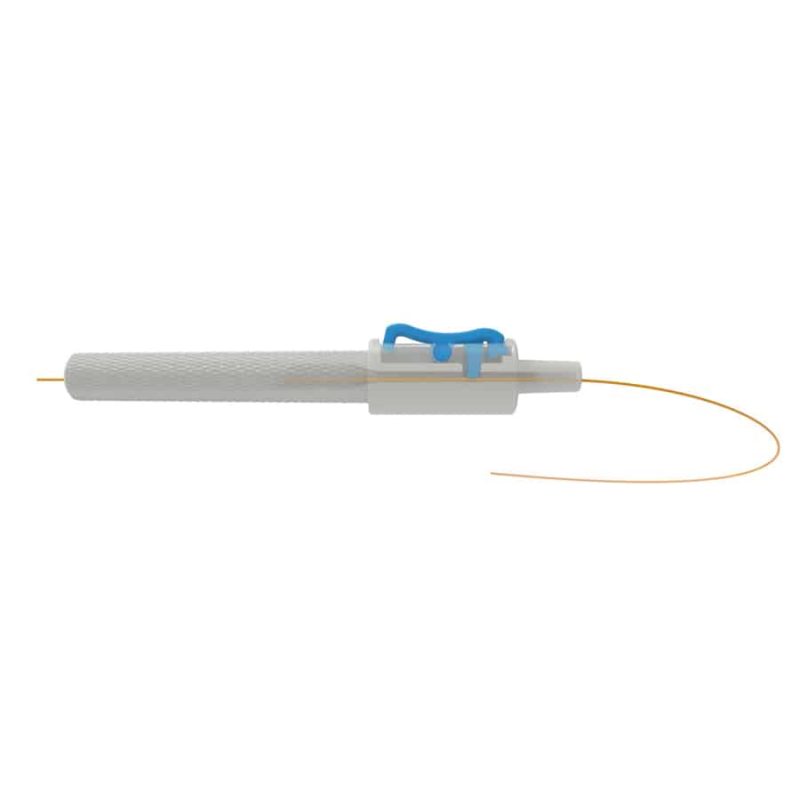
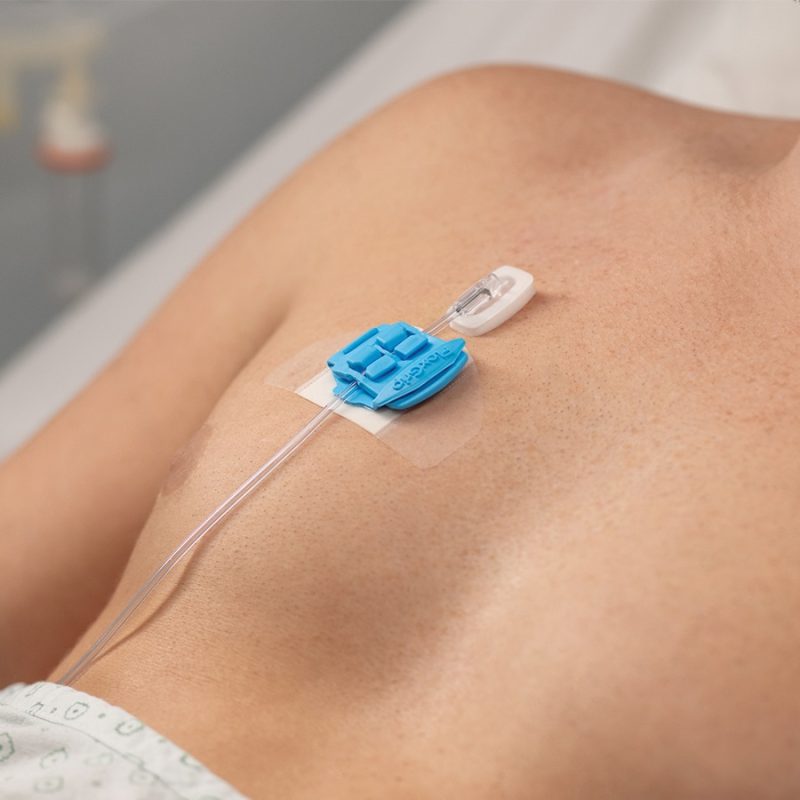
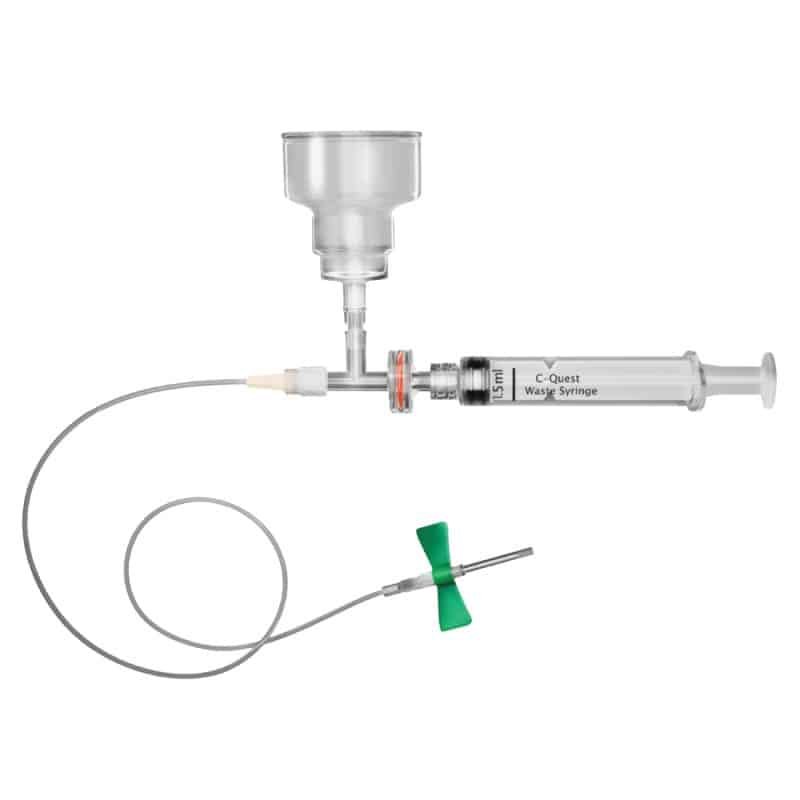
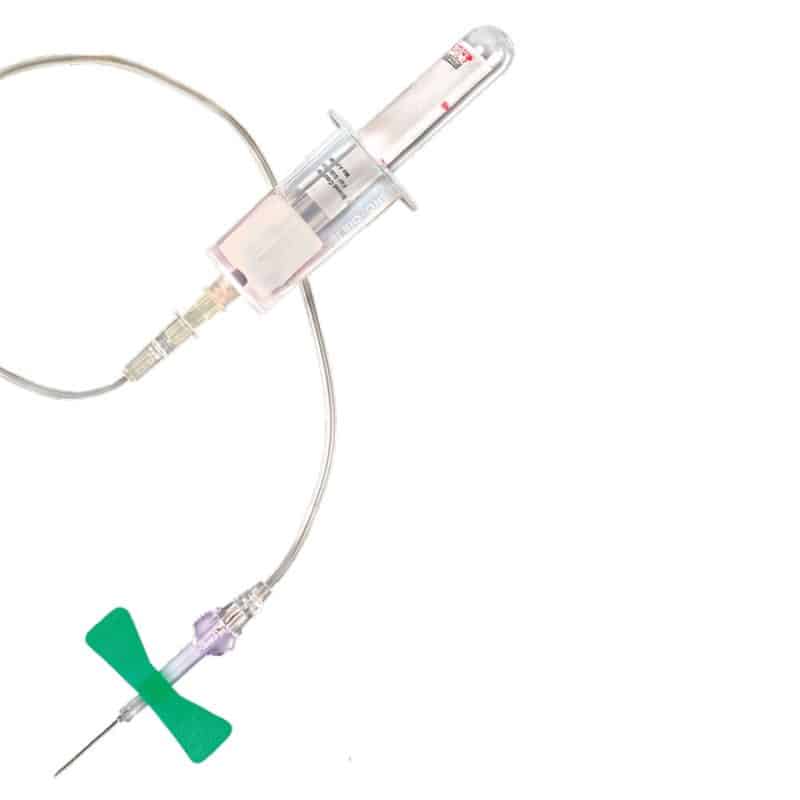
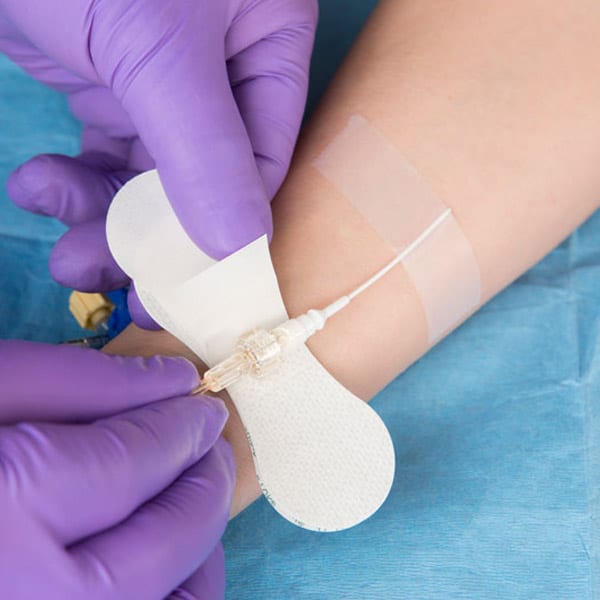
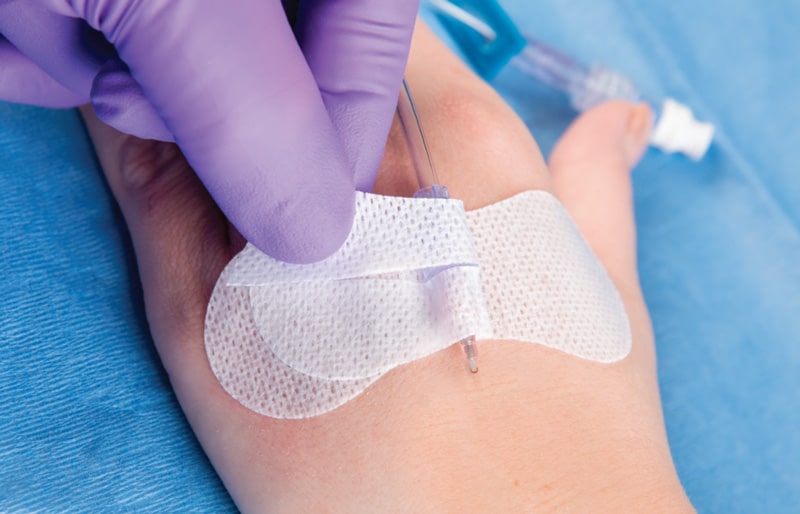
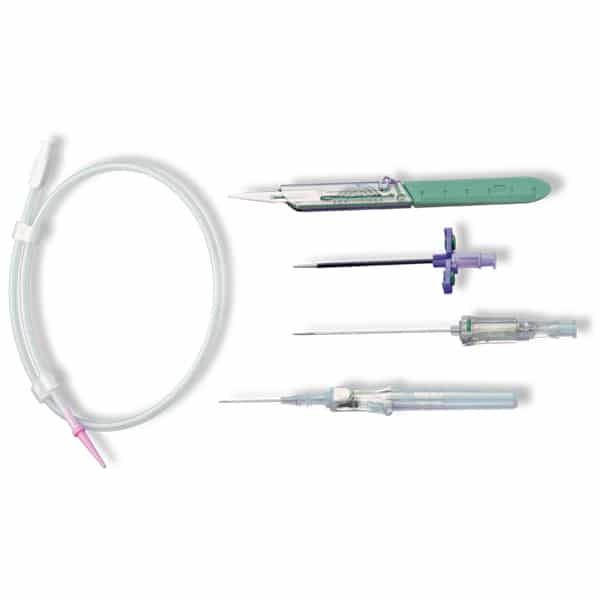
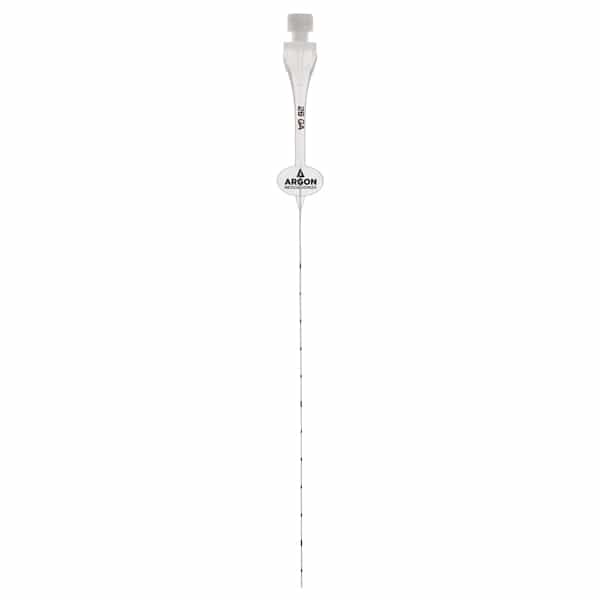
Description
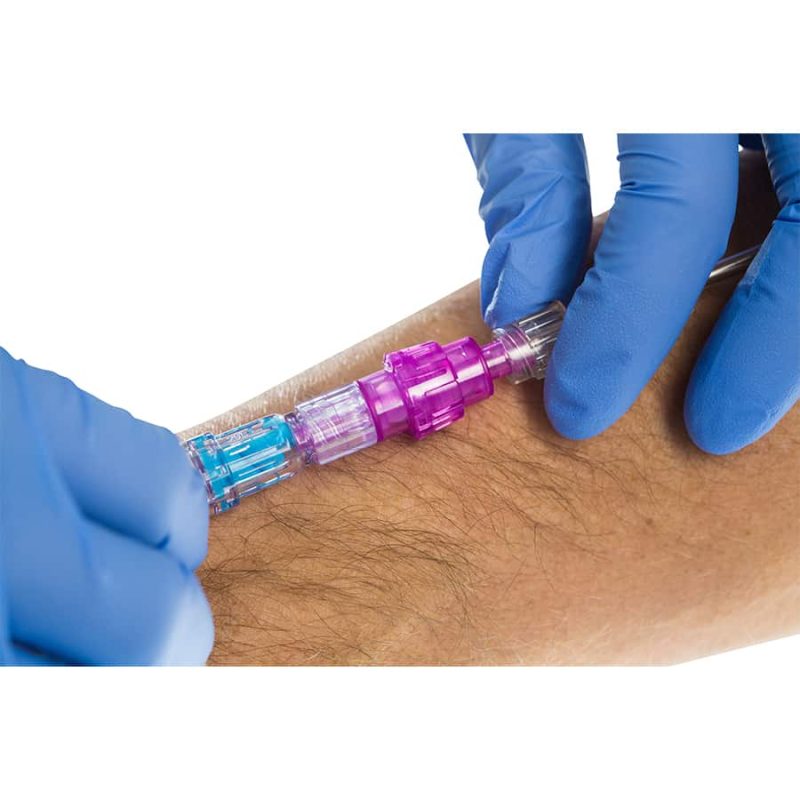
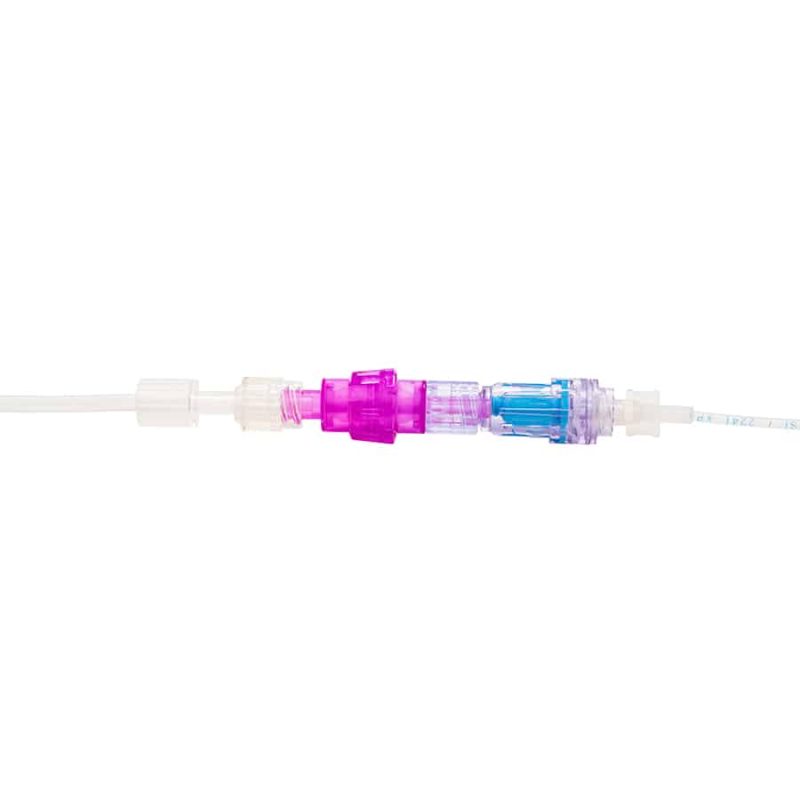
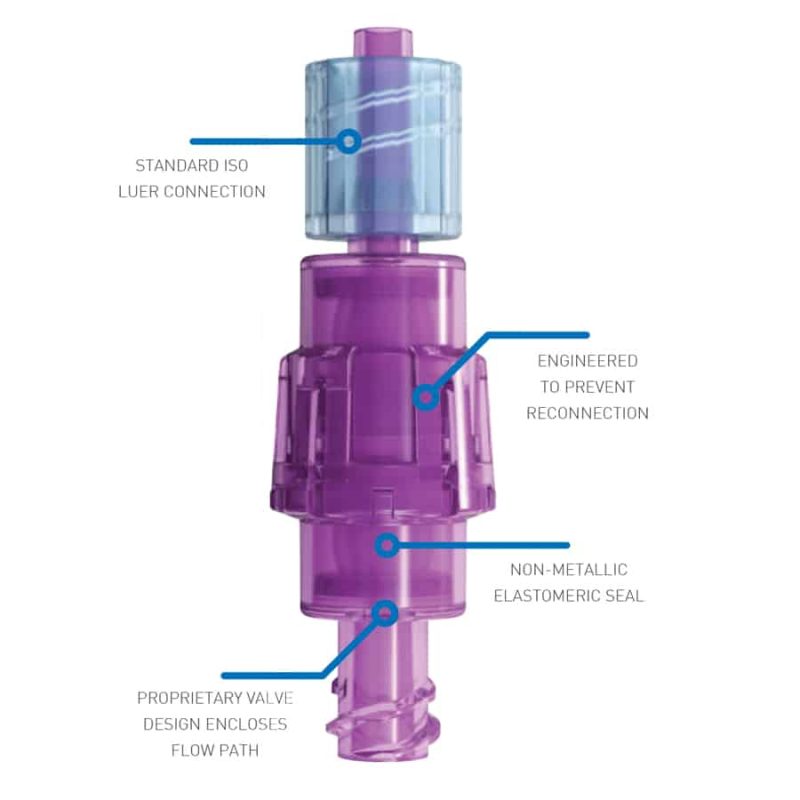
Benefits of the Orchid SRV
- Avoid unscheduled IV restarts
- Decrease exposure to Sharps Injuries
- Retain continuity of care
- Achieve greater patient safety and satisfaction
- Focus on value-added nursing time
- Save valuable hospital funds
Contact us to utilize the Orchid SRV Hospital Savings Estimation Calculator and schedule a demo.
The Orchid SRV is on contract with:
- Vizient Contract #MS7242.
- Premier Contract #PP-IV-155
- VA Federal Supply Schedule (FSS)
References:
- iData research report
- “The Peripheral Intravenous Catheter Journey: A Prospective Cohort Study of 1000 Patients.” Podium presentation by Nicole Marsh, RN, and Claire Rickard, RN, PhD, AVA 2017 annual meeting
For more information about the Orchid Safety Release Valve from Linear Health Sciences, contact your local MED Alliance Sales Representative, call 888-891-1200, or email us.