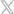Aligning with the Newest INS Guidelines: The Orchid Safety Release Valve (SRV)
The 2024 Infusion Therapy Standards of Practice (9th Edition) from the Infusion Nurses Society places more emphasis than ever on site protection and stabilization, specifically dislodgement.
Standard 37.1 addresses the need to safeguard vascular access devices (VADs) “from patient manipulation, inadvertent dislodgement, and exposure to contaminants.” The recommendations also mention, in 35.1 A, the use of add-on devices such as safety release valves/connectors to decrease movement/manipulation.
The standard emphasizes the importance of adopting evidence-based solutions to protect vascular access and improve outcomes.
IV dislodgements are more than an inconvenience; they can compromise patient safety, delay treatment, and increase complications such as infiltrations, phlebitis, and occlusion.
A Smarter Solution: The Orchid™ SRV
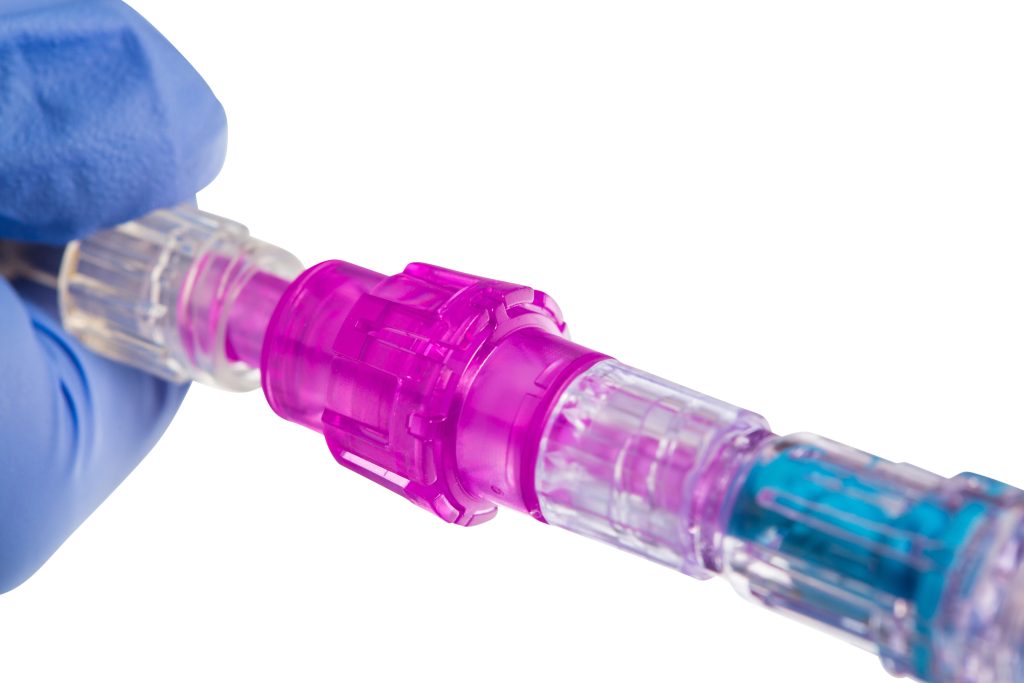
That’s where the Orchid Safety Release Valve™ (SRV) from Linear Health Sciences™ plays a vital role. The Orchid SRV is a solution designed to prevent IV dislodgements and minimize unnecessary restarts. The sterile, tension-based device is placed between the main IV administration set and extension set, as well as on all vascular access catheters. The SRV automatically disconnects under minimal force and seals both sides to create a sterile barrier. The device can be used in conjunction with securement dressings, aligning with practice recommendations.
The Orchid SRV’s simple design helps prevent inadvertent dislodgement, minimizes catheter movement, enhances patient safety, reduces the need for IV restarts, and supports compliance with the updated INS standards.
Benefits of the Orchid SRV
- Reduce IV dislodgements and avoid unnecessary restarts
- Enhance patient safety and satisfaction
- Minimize exposure to sharps injuries
- Save valuable hospital resources
- Free up nurses for more value-added care
To learn more about the Orchid Safety Release Valve or to request an evaluation, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.