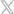Safely and Efficiently Preserve HPCs with Cell Freeze®
The Cell Freeze® cryogenic storage containers from Charter Medical are specifically made to efficiently store, preserve, and transfer hematopoietic progenitor cells (HPCs).
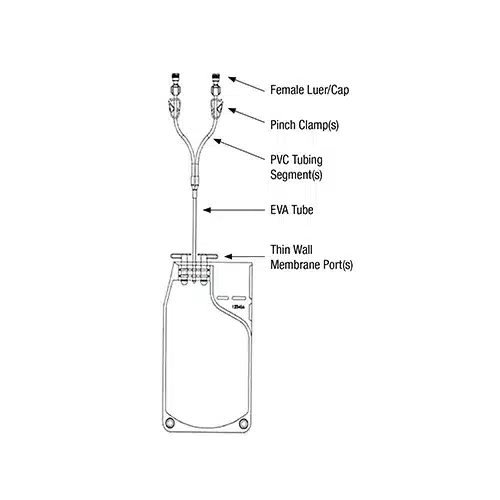
Cell Freeze® Features and Benefits
Each Cell Freeze® bag is rated for storage in liquid nitrogen to -196°C and individually packaged in a Tyvek pouch. The bags are manufactured with a material that is made of resistant polyolefin film material that maintains flexibility when frozen and features an integral fill tube that eliminates the need for PVC interferences with the liquid nitrogen storage section of the container. Each bag features two membrane access ports with thin walls to enhance bag flexibility to accommodate complex freezing cycles. The attached cap minimizes membrane exposure during freezing. Each bag is equipped with an industry standard label pocket design, offering ease of use and traceability within labeling.
Physical Integrity Testing
Cell Freeze® bags were designed to support routine storage of hematopoietic progenitor cell products. The physical integrity of the bags was successfully tested for durability under typical applicationsinvolving temperature variations. The physical integrity tests performed include pressure leaks, microbial challenge and dye immersion.
Cell Quality
Cell Freeze® bags were assessed on cell quality using diluted HPC with 10% DMSO. Conclusions of the study reflected that all containers met the acceptance requirement for MNC and CD34+ cell recovery of ≥ 70% relative to cell counts of the sample prior to cryopreservation. “The average
cell recovery for MNC was 81%, and the average cell recovery for CD34+ was 84%. All containers met ≥ 1 CFU acceptance criteria with an average of 78% recovery.
To learn more about the Cell Freeze®, please call 888-891-1200 or email us.
MED Alliance Group is a medical device distributor with more than 350 years of combined medical device sales and distribution experience. Dedicated to meeting the needs of its clinical customers and manufacturing partners, MED Alliance offers cost-effective, customized sales, logistics, and distribution solutions.