Reduce the Risk of Pediatric PIVC Failure with the Orchid Safety Release Valve
Surement Alone is Not Enough: Pediatric PIVC Dislodgement is Common But Can Be Reduced
When pediatric patients are admitted to the hospital, it is estimated that 80% require peripheral intravenous catheters (PIVCs) to administer fluids, blood products and/or medications[1]. Although securing a PIVC is a common procedure, it isn’t quick or easy, especially when the patients are scared and agitated children with very small veins.
Studies indicate only 40%-50% of first-attempt pediatric PIVC placements are successful, typically taking 20-30 minutes.[2] Typically a two-person job, the procedure is time-consuming and can be traumatic and painful for patients.
Unfortunately, reports show that PIVC failure is common in pediatric patients, even with dressings and securement. One study found complications in 40.9% of its pediatric PIVCs, with 63% of the failures caused by dislodgement.[3] Another study shared that 25% of its pediatric PIVCs failed, with 5% due to dislodgement.[4]

A Known Concern
Dislodgement is a known concern that the vascular access community has been trying to tackle for decades with engineered securement devices.
A study published in JAVA surveyed more than 1500 clinicians who reported that 50% of all IVs still dislodge even with extra securement. Of those surveyed, 58% reported seeing accidental dislodgements daily or often and 68% felt dislodgement is a safety risk.[5]
The Costs of IV Dislodgements
- Delay in patient treatment and procedures
- Increase in patient complications
- Medication waste
- Consumables to restart the IV
- Nursing time
- Increase in exposure to sharps injuries
Preserving Pediatric PIV Catheters: The Orchid SRV™
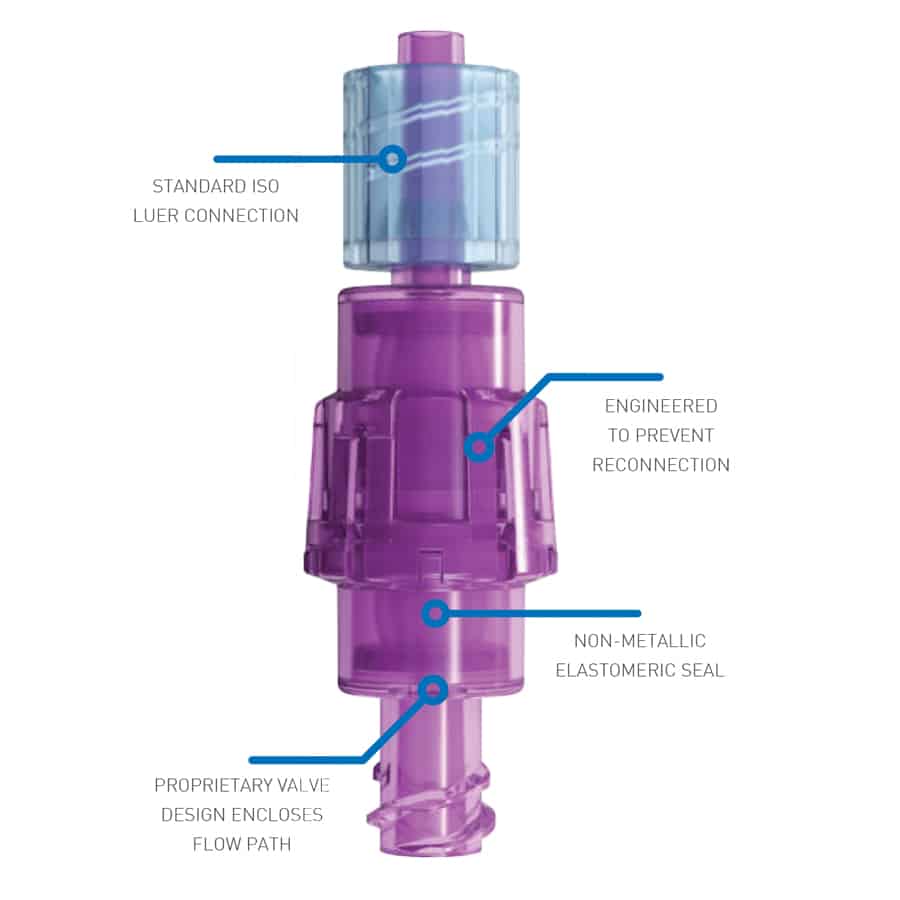
The Orchid SRV (Safety Release Valve) from Linear Health Sciences is a tension-activated breakaway safety release valve designed to reduce the risk of IV catheter failure and replacement in patients two weeks of age and older.
The Orchid SRV is a sterile, single-patient use connector for needle-free access, placed between the existing IV extension set and general IV tubing connection intended to be used for delivery of fluid to and from the IV catheter.
The device can be used during direct injection, intermittent infusion and continuous infusion. When tension across the device exceeds a predetermined threshold, the device separates into two halves, activating valves that create sterile seals for the fluid pathway, with the goal of retaining the IV site integrity.
The Orchid SRV makes return to treatment fast, simple and clean. No special training is required. When used, nurses can focus on value-added nursing time and achieve greater patient safety and satisfaction.
Every Line. Every Time.
Break away from the expense and pain of IV dislodgements. The Orchid SRV helps to:
- Retain continuity of care
- Avoid unnecessary IV restarts
- Decrease exposure to Sharps injuries
- Achieve greater patient safety and satisfaction
- Focus on value-added nursing time
- Save valuable hospital funds
Click here to learn more.
To learn more about the Orchid Safety Release Valve or to request an evaluation, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1757963/
[2] https://onlinelibrary.wiley.com/doi/full/10.1111/1742-6723.12305#emm12305-bib-0004
[3] https://www.researchgate.net/publication/347136466_Predicting_factors_for_complications_in_peripheral_intravenous_catheters_in_the_pediatric_population
[4] https://onlinelibrary.wiley.com/doi/full/10.1111/1742-6723.12305
[5] https://medalliance.showpad.com/share/8MPQpDOyxYYD6Y9HtRlOH