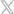68% of Clinicians Report Seeing IV Dislodgements “Often, Daily and Multiple Times a Day”
IV Dislodgements Pose a Safety Risk to Patients, Waste Hospital Resources
In a study published in the Journal of the Association for Vascular Access, 68% of the more than 1500 clinicians surveyed reported seeing IV dislodgements “often, daily and multiples times a day.”
Each year, more than 70-90% of acute care patients in the United States receive an IV. Even with catheter securement and dressings, IV dislodgement rates are at 1.8 to 24%.
95% of the surveyed clinicians agreed that IV dislodgements pose a safety risk to patients.
Dislodgements can lead to patient complications, delays in treatment, increased nursing time and medication waste – costing an estimated $5 million each year.
Click here to read the study in full.
Catheter securement has come a long way, but more should be done to prevent IV dislodgements.
Solution: The Orchid Safety Release Valve
Study Shows the Orchid SRV Prevents IV Dislodgement by 91.9%
The Orchid SRV™ (Safety Release Valve) from Linear Health Sciences is a sterile, single-patient-use connector for needle-free access, placed between the existing IV extension set and general IV tubing connection intended to be used for delivery of fluid to and from an IV catheter.
The Orchid SRV can be used during direct injection, intermittent infusion, and continuous infusion. When tension across the device exceeds a predetermined threshold, the device separates into two halves, activating valves that create sterile seals for the fluid pathway, with the goal of retaining IV site integrity.
A clinical simulation study showed the Orchid SRV prevented IV dislodgement by 91.9% across all test groups in which the device separated, and no dislodgement occurred. Click here to read the White Paper.
Every Line. Every Time.
Break away from the expense and pain of IV dislodgements. The Orchid SRV helps to:
- Retain continuity of care
- Avoid unnecessary IV restarts
- Decrease exposure to Sharps injuries
- Achieve greater patient safety and satisfaction
- Focus on value-added nursing time
- Save valuable hospital funds
Click here to learn more.
To learn more about the Orchid Safety Release Valve or to request an evaluation, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been dedicated to meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.