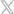Is Your Immunization Program Ready for COVID-19 Vaccines?
Will You Need to Transport the COVID-19 Vaccine?
Immunization programs across the country are gearing up for the ultra-cold, frozen and refrigerated COVID-19 vaccine distribution.
According to the CDC’s “Vaccine Storage and Handling Toolkit” and the recently added “COVID-19 Vaccine Addendum,” it is essential for each vaccine unit / container to have a temperature monitoring device.
Many healthcare locations will need to transport the COVID-19 vaccines to multiple sites (on-campus buildings, remote location, curb-side, etc.) while ensuring temperature stability.
MaxPlus Vaccine Coolers with MaxTrace™ temperature monitoring technology are a solution, capable of transporting thousands of doses (based on Pfizer initial estimates) at refrigerated (2-8° C, thawed and diluted) temperatures.
Learn more by reading a whitepaper summarizing recent COVID-19 vaccine developments, CDC guidelines and vaccine manufacturer instructions.
Click here to view all of the MaxPlus Vaccine Coolers.
Overview of MaxPlus Vaccine Coolers with MaxTrace™ Technology

AV12X12
Size: 13.5″x13.5″x13″
Payload: Refrigerated vaccines
Payload Dimensions: 5.75″x10″x8″
Payload Temp: 2 – 8° C
Coolants: Refrigerated and frozen reusable coolant bottles
Validation: Up to 12 hours of storage or transport at controlled ambient temperatures
MaxTrace™ Temperature Monitoring System

AV18X12
Size: 18″x12.5″x14″
Payload: Refrigerated vaccines
Payload Dimensions: 11″x10″x7″
Payload Temp: 2 – 8° C
Coolants: Refrigerated and frozen reusable coolant bottles
Validation: Up to 12 hours of storage or transport at controlled ambient temperatures
MaxTrace™ Temperature Monitoring System
For more information on MaxPlus Vaccine Coolers, call 888-891-1200 or email us to be connected to your local sales representative.
MED Alliance Group is medical device distributor that has been dedicated to meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, as well as NICU and PICU.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.